HepaSH Sustainable Human Hepatocytes
Powered by CIEM
Marketed WW by Biopredic International
HepaSH Features
- Sustainability
- Consistency
- Robustness
- Functional performance
- Long-Term Viability
- Multidonor set
- Zero stock policy
- Data base
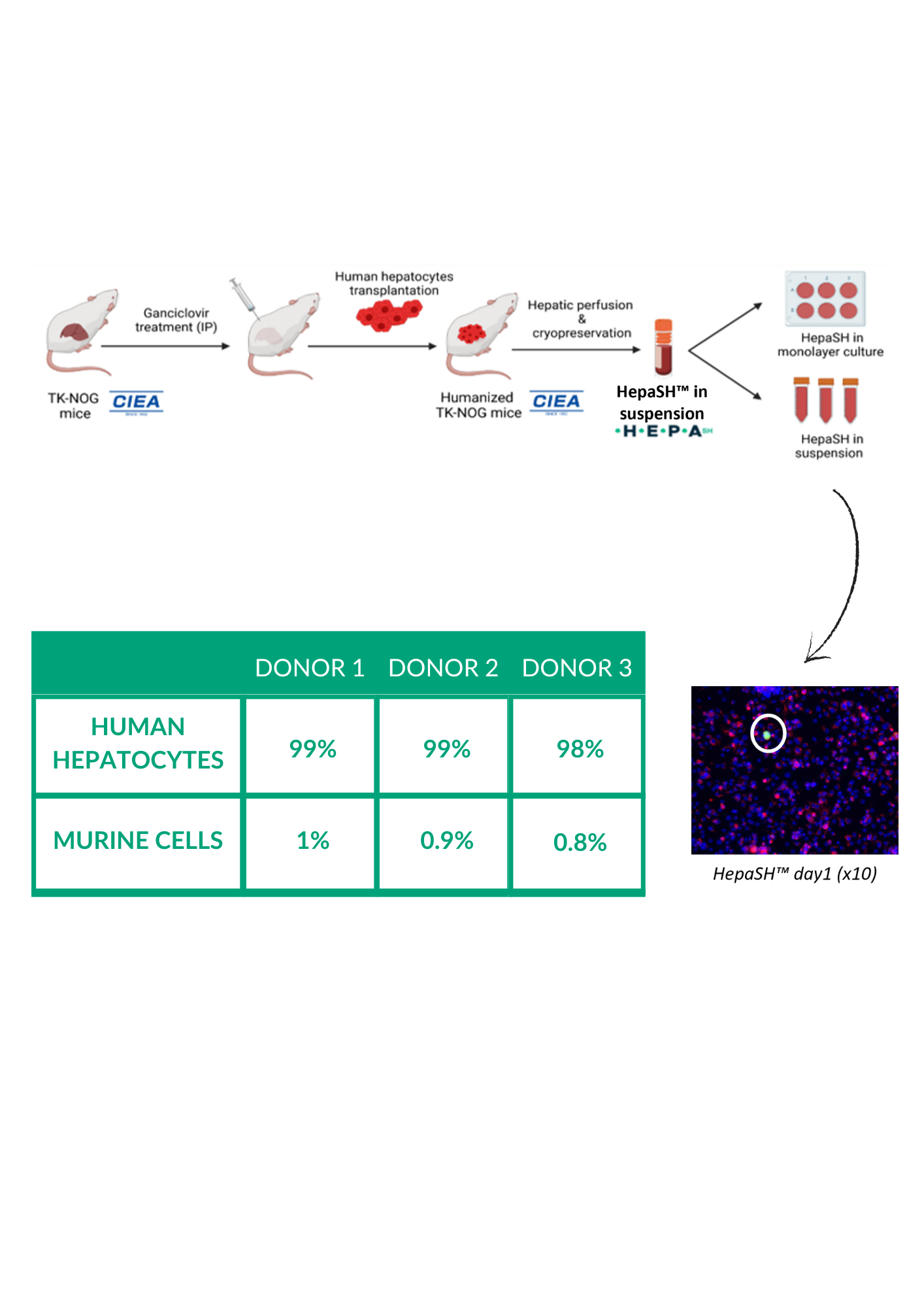
HepaSH Standardization Technology
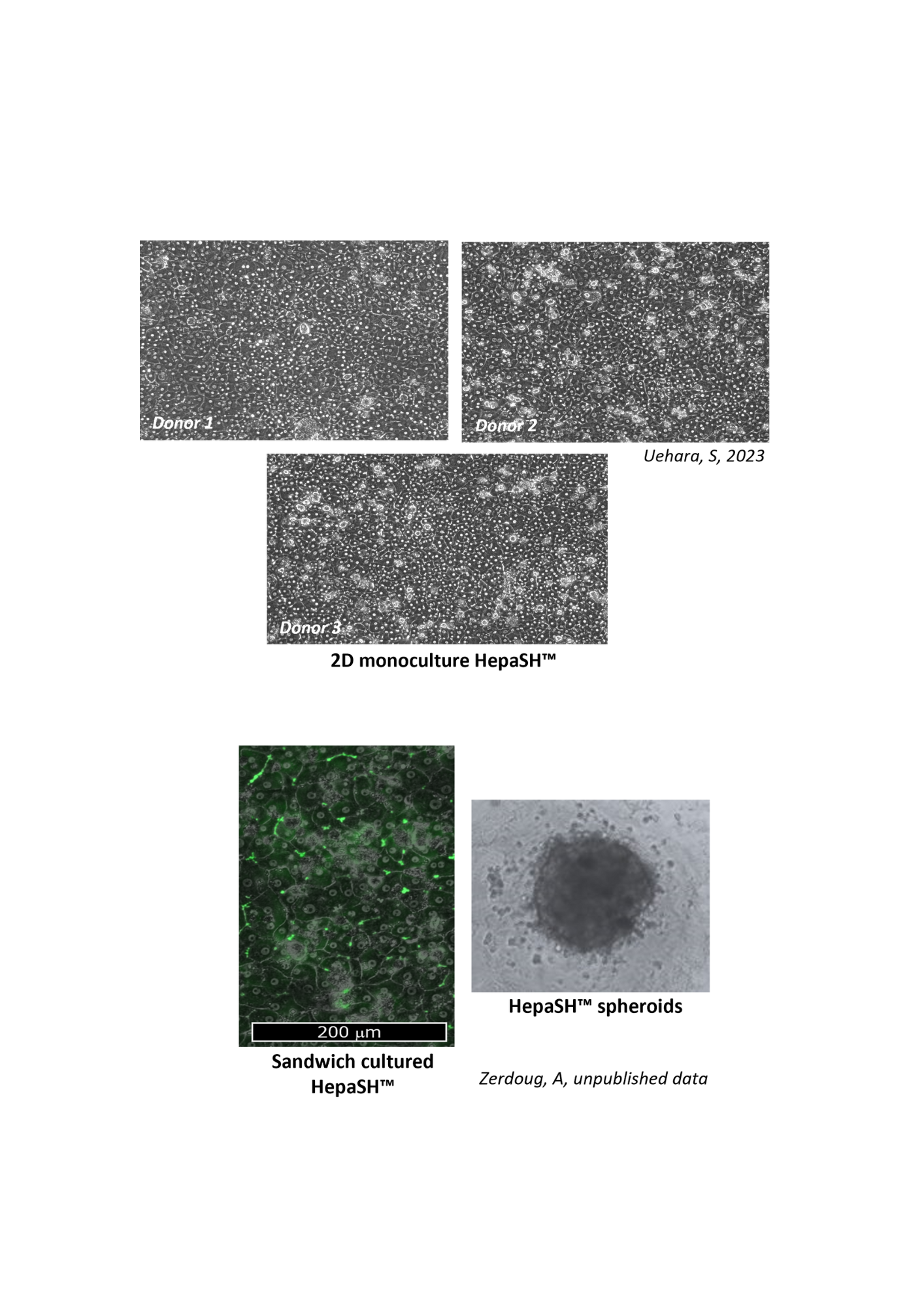
HepaSH Morphology and Life Style
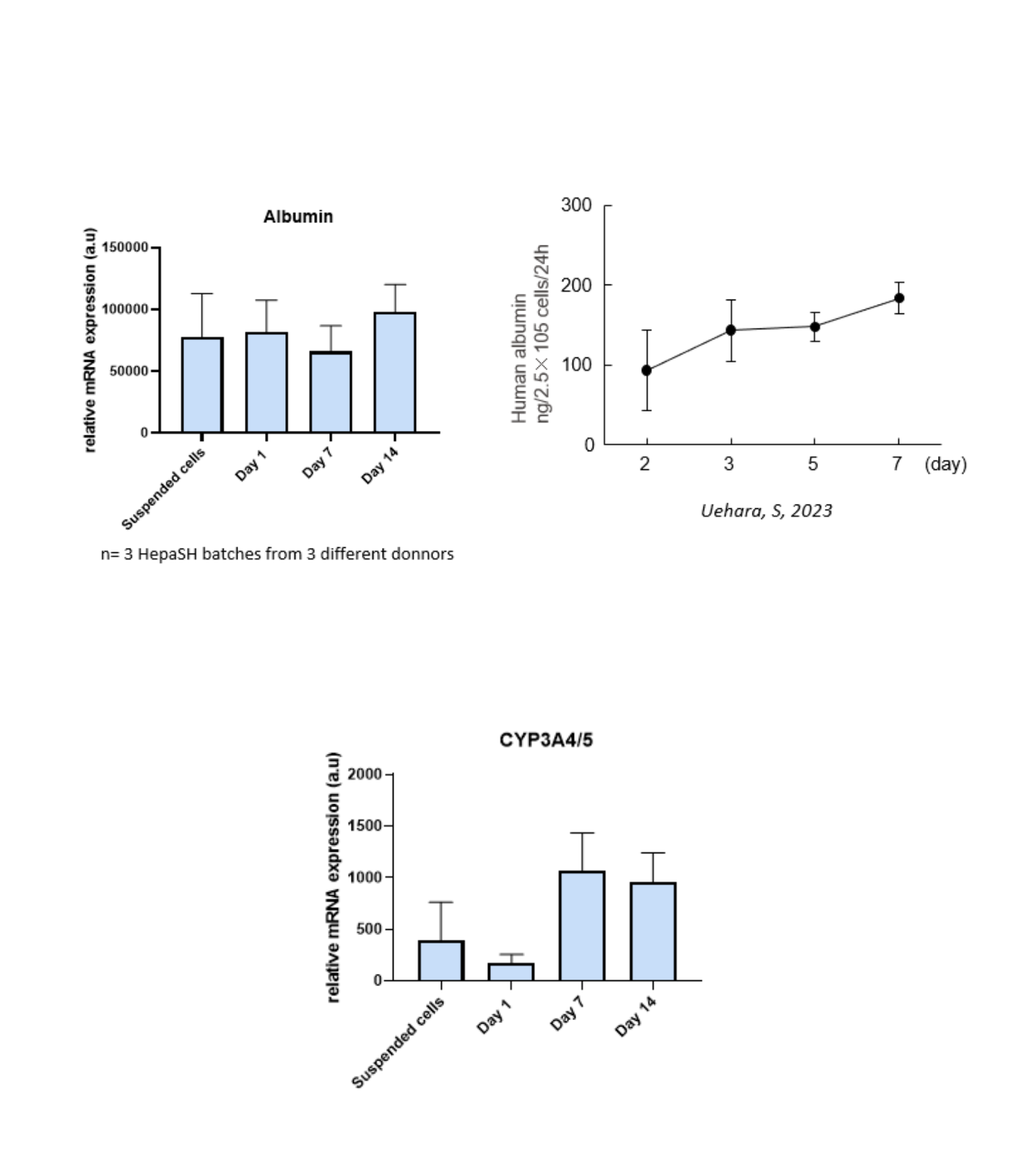
Hepatic markers expression
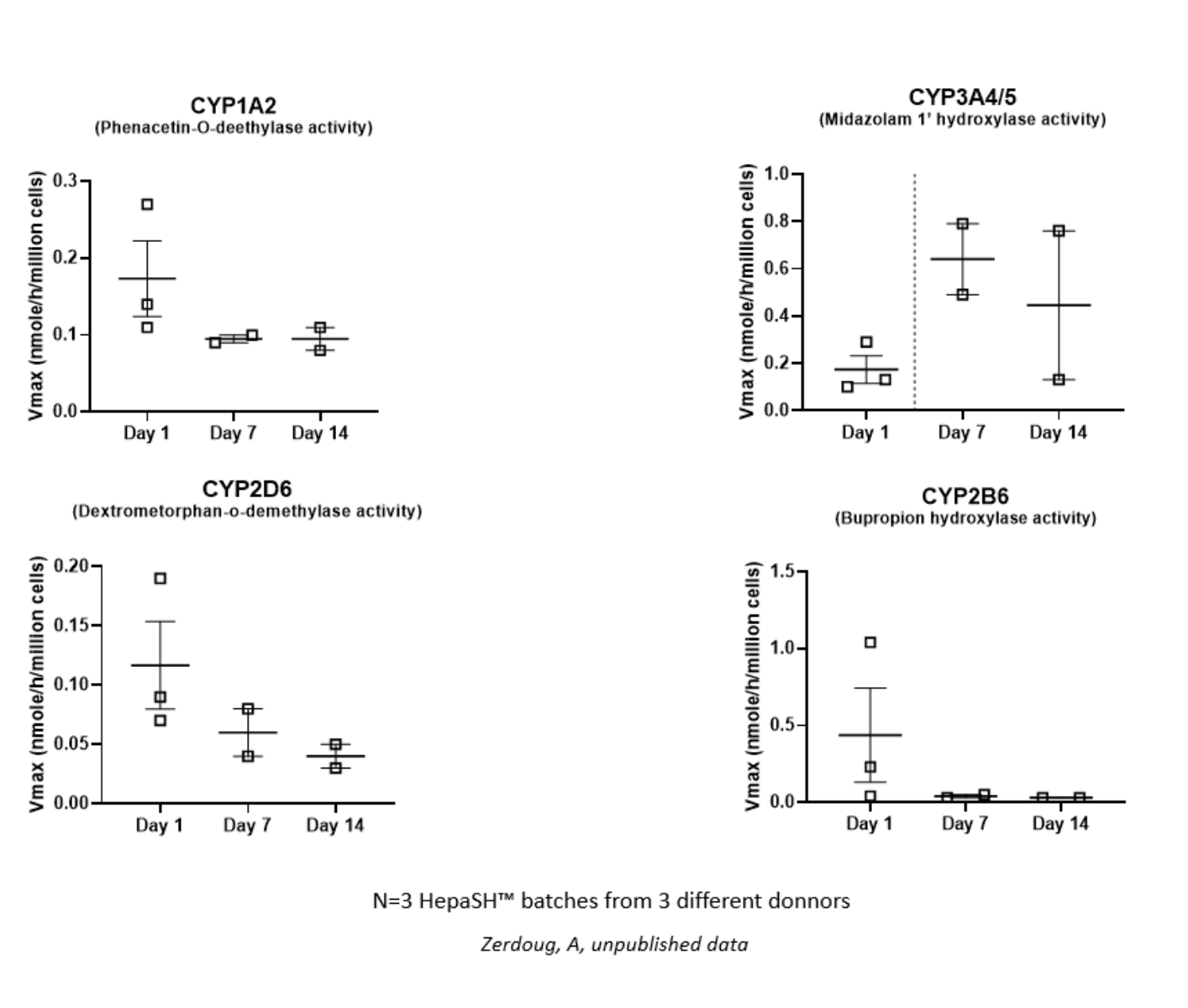
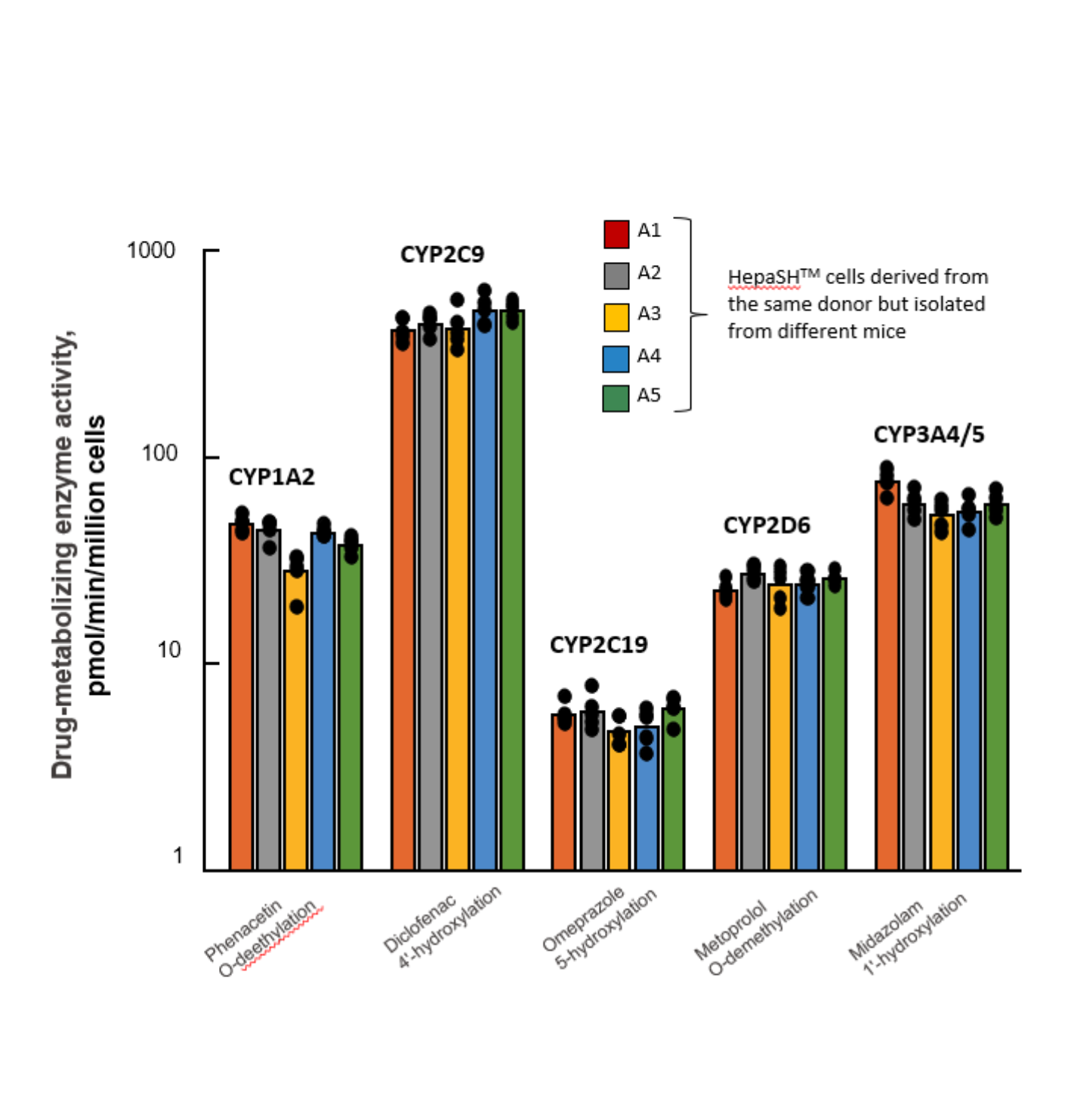
HepaSH Metabolically Competent HH
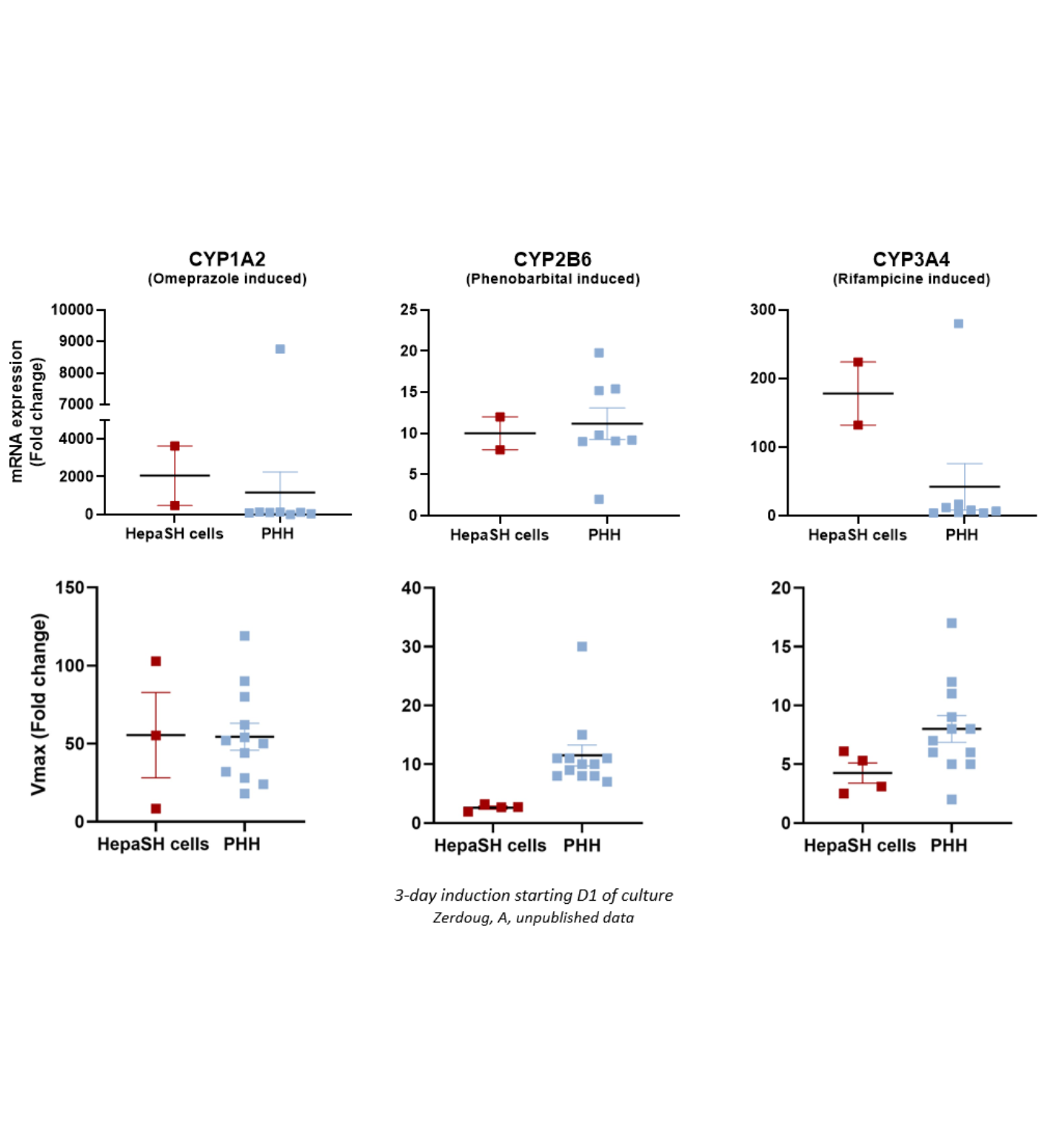
HepaSH CYP Inducibility
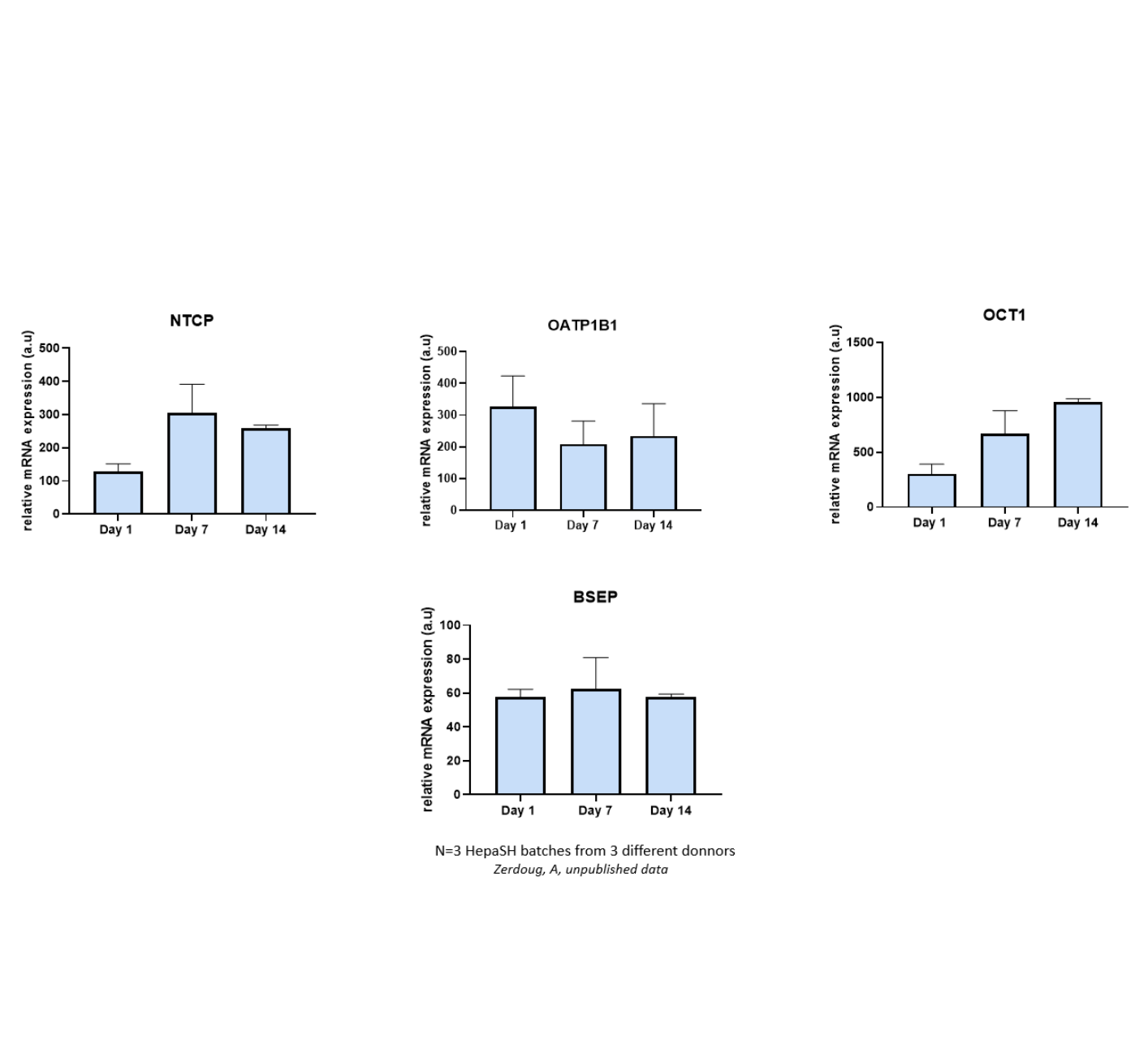
Transporters
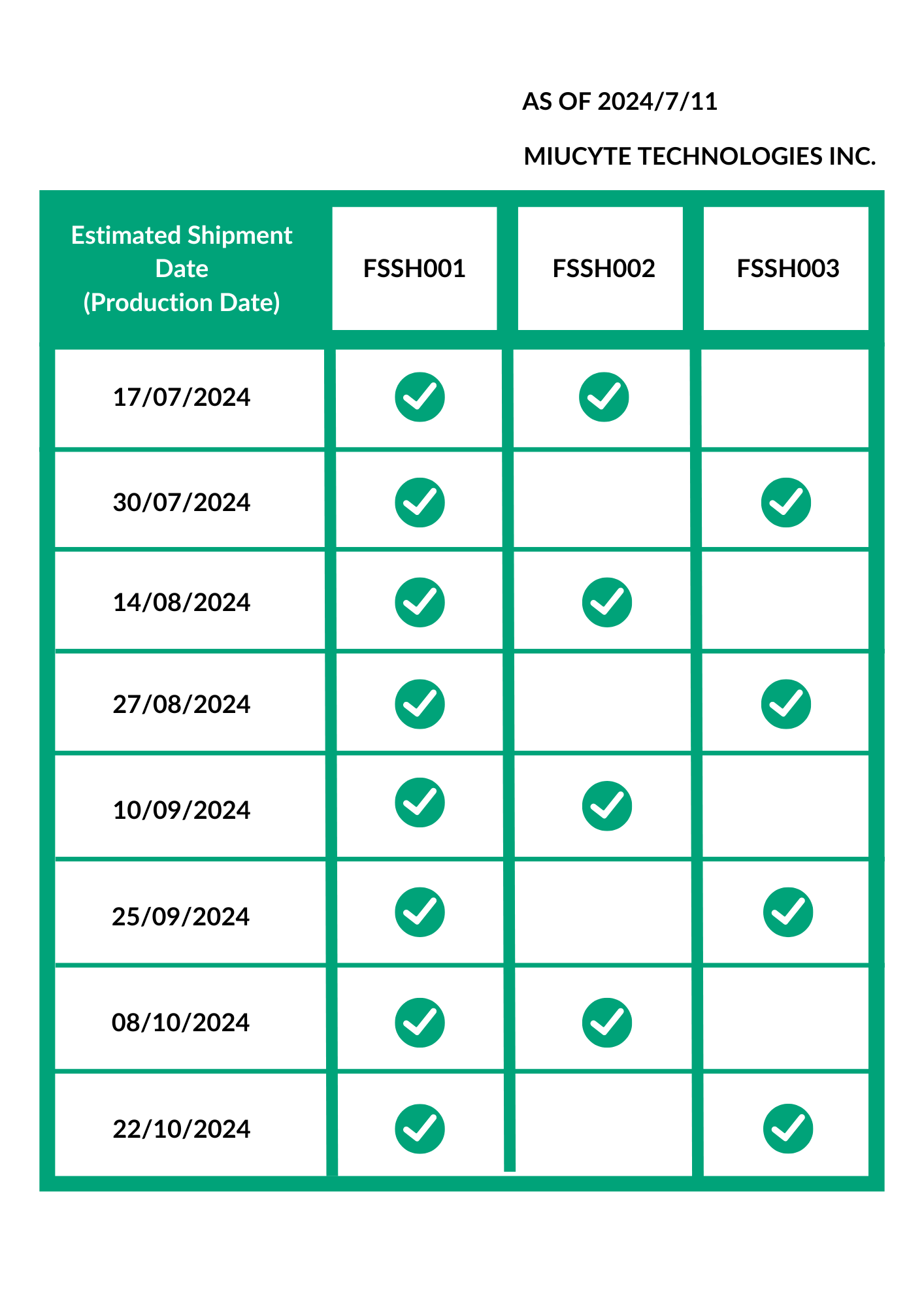
HepaSH Production and Shipment Schedule
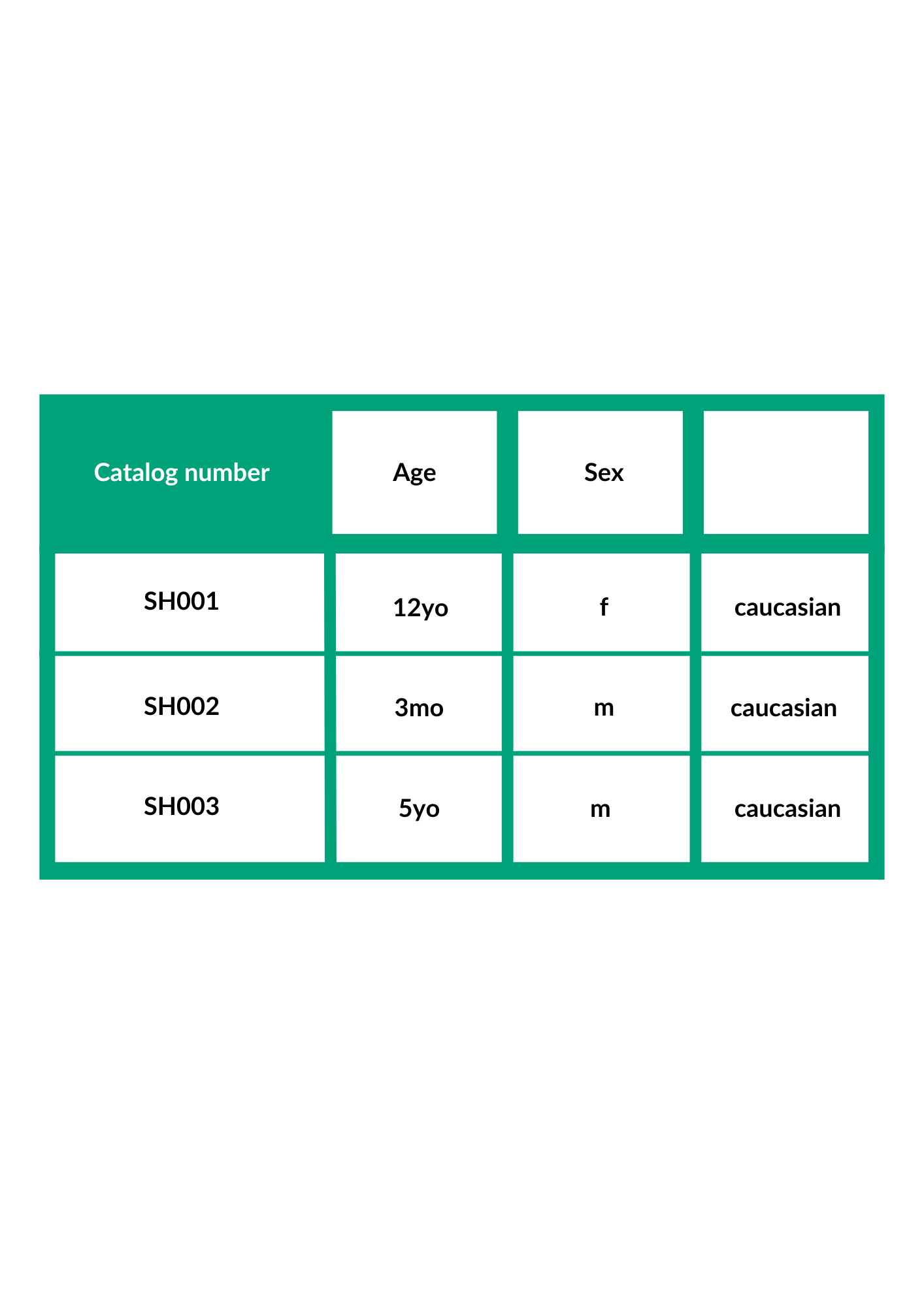
HepaSH Donor Information
Fresh Suspended Ready-to-plate HepaSH
FSSH001: 5.106 cell/vial 1x 96-multi well plate
-
Provided as fresh suspended hepatocytes
-
3 donors available as of now
Donor 1: Female, Caucasian, 12 years old. Donor 2: Male, Caucasian, 3 months. Donor 3: Male, Caucasian, 5 years old -
William E- based cell culture medium
-
Can be maintained for up to 14 days in 2D culture
-
2 production sessions/month
-
Our commitments: cell viability, number of viable cells, human cell percentage, sterility
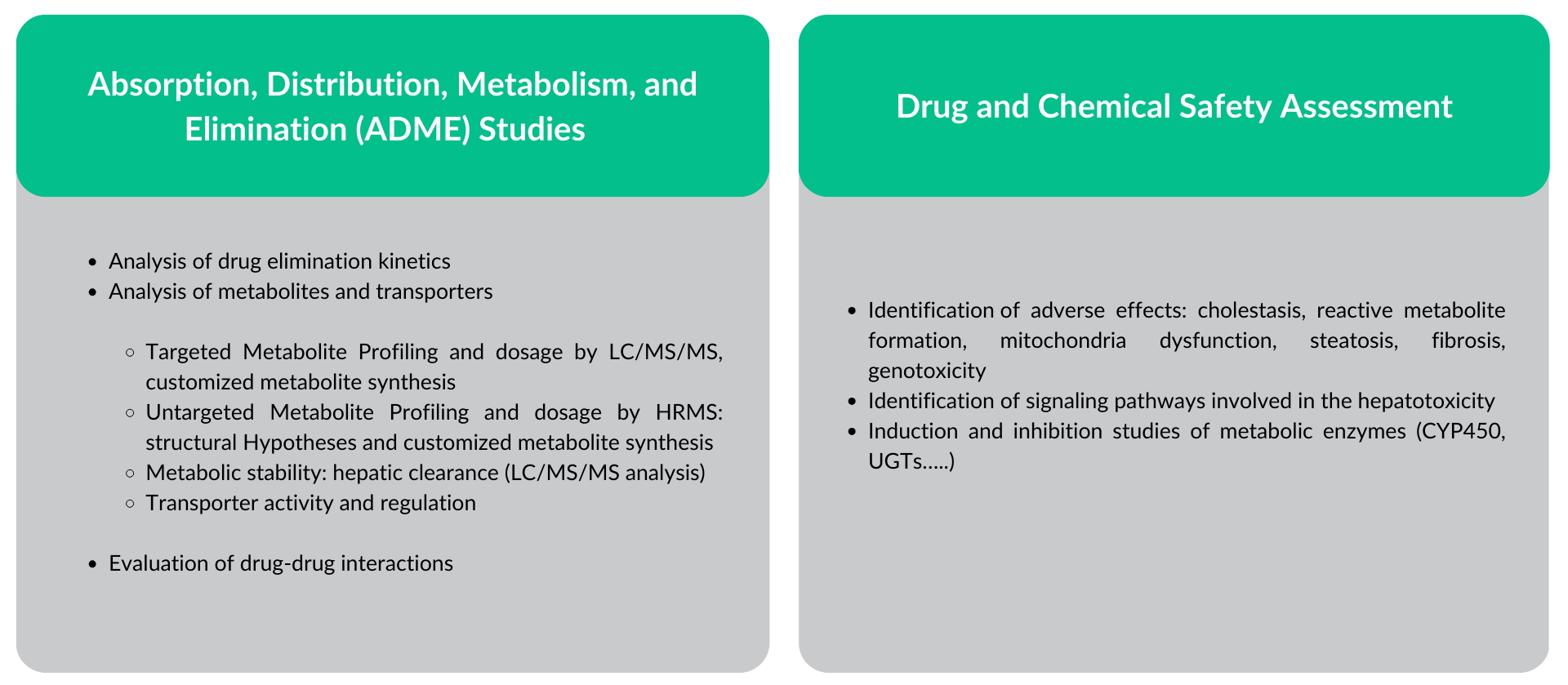
Our services on HepaSH human hepatocytes
News
EUROSAFE
Mar 25, 2022
Un nouvel espace de travail Wepredic !
Biopredic International et Eurosafe se réunissent au sein de la même structure avec un tout nouvel espace de travail.
EUROSAFE
Mar 21, 2022
Using transcriptomics in toxicology: toxicogenomics
Transcriptomics data are relevant to address several challenges in toxicology. The toxicogenomics data can be used in predictive toxicology, where more advanced modeling techniques are applied.
WEPREDIC
Mar 14, 2022
BPI/France, UL/Lebanon, FHNW and Edw/Switzerland, join their efforts on NAMs for a Safer World
In our long-standing contribution to improving product safety and drug toxicity predictivity for a Safer World, Biopredic International (BPI) organized, with its collaborators from the Lebanese University (UL)…