Safety & bioavailability Assessment services

We provide expertise and new approach methodologies, for testing cosmetics, chemicals, pharmaceuticals medical devices and food supplements.
Our 4 ranges of safety and bioavailability assessment sevices
We do not sell tests as such. We work with you on making your product compliant, safe, pleasant. We sustain your claims and your customer’s trust.
-
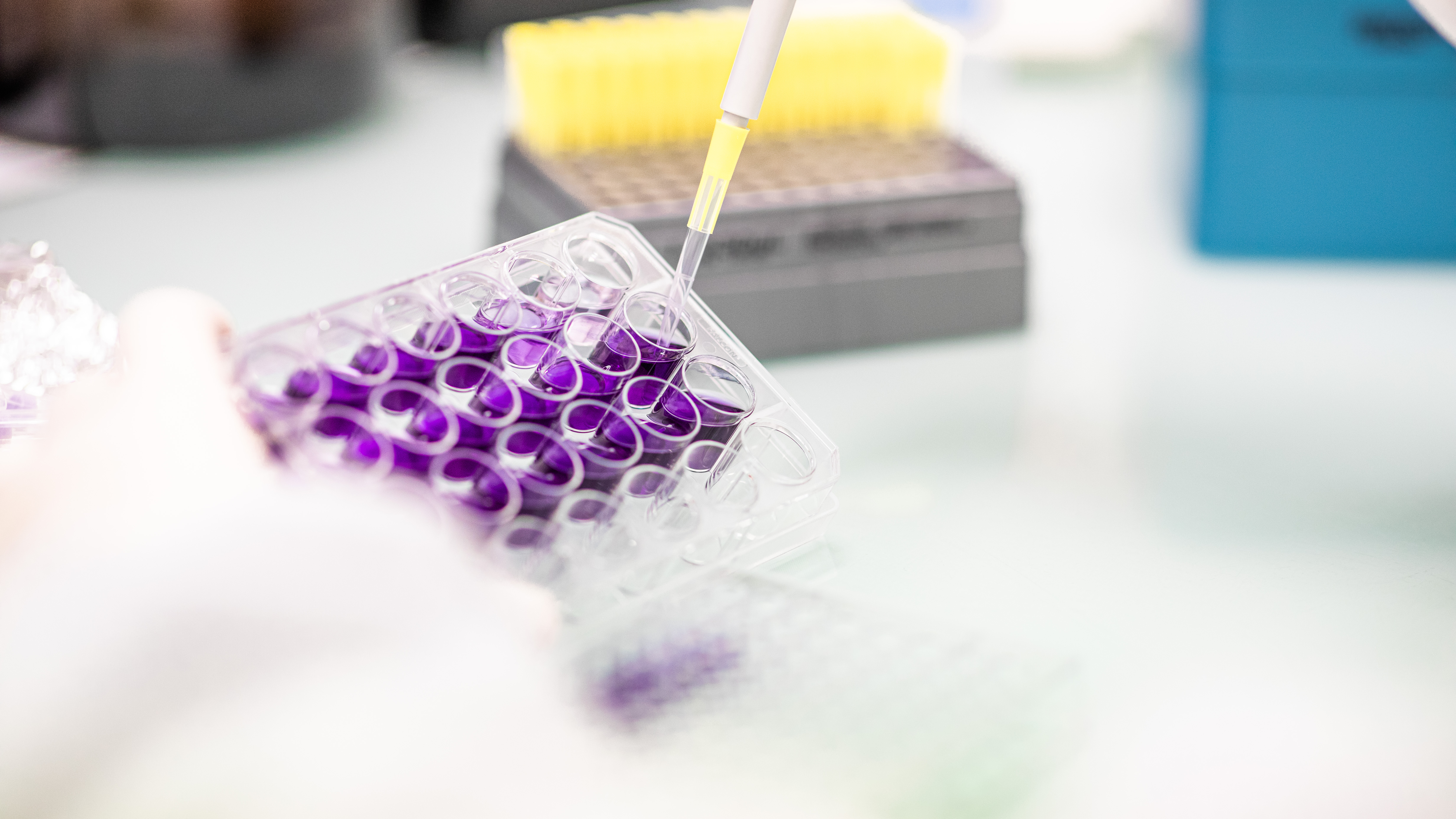
In Vitro Assay
-
Test on volunters
-
Toxicological & regulatory expertise
-
In silico modeling
Eurosafe is inspected by the French Drug Agency in matter of Good Laboratory practices compliance.
Did you Know ? EU companies can obtain funding for up to 30% of your research and development expenses.
Our latests offsprings

Cosmetics
SensisTM
The Sensis assay, licensed by Immunosearch asesses the potency of the sensitization potential of a chemical ingredient and finished cosmetic products. The Sensis assay is performed in a 3D skin system with a genomic signature readout. This genomic potency slassification makes the Sensis assay unique in its prediction of risk assessment. Moreover, the Sensis assay takes into account the metabolites of the chemical(s). And, its domain of application is the largest on the market, incliding non-water soluble compounds and complete formulation.
MICROBIOME
Developped with Vaiomer to verify that a product does not alter the skin microbiome.

Drugs
Drug-Drug interactions / Metabolic Stability / Species Comparisons / Metabolite Identification / Profiling Drug Transporter substrate & inhibition
Novel assays
Liability screening of cholestatic drugs with a unique patented assay relying on the bile canaliculi function and morphology
Measurement of the fraction metabolized value, CYP per CYP, for a new compound.
How to book assessment services
You can purchase our products and services either
They trust us and in our products
We are working XX



